One of the major questions that comes to mind at the procurement stage for oral-care brands is “Who will make the product?” Will it be OEM, white-label, or contract manufacturing? The manufacturing model you choose determines who owns the product’s IP, who pays the setup costs, how fast the product reaches the shelves, and who bears the regulatory and packaging risks.
For oral care brands, picking the wrong type of manufacturer can lead to delays in product listing, warranty claims, or even delisting. In this guide, we covered the trade-offs, technical triggers, and compliance checkpoints to help you select the right manufacturing type.
Keskeiset tiedot
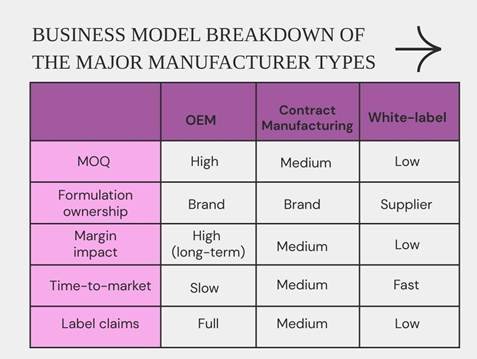
- OEM: You pay for product development while the manufacturer makes your exact formula and packaging. We often advise clients to choose OEM when exclusive claims, full control, and long-term margin are a priority.
- White-label / Private label: This model is cost-effective, quick, and ideal if you want a fast launch with little upfront cost. Low-volume orders and simple products are often manufactured through this model.
- Contract manufacturing/Contract filling: You own the formula while the manufacturer fills and packages it. Pick contract fill if you already have a tested formula but no factory.
Why You Should Choose Between OEM vs White-Label vs Contract Manufacturing
- You have to pick a model that matches your business stage. We had a startup client with only $5K R&D budget and a 6-week launch window, so speed and low cost were paramount. Established brands planning to scale need to protect their IP and have margin control.
- Business economics change dramatically when it comes to MOQs, so you need a model that works well with your volume forecast.
- To patent your product claims, we recommend a model that guarantees formula control and full validation. For instance, mouthwash works best on formulation IP and flavor stability, so you need a mouthwash manufacturing model that ensures product consistency.
- How fast do you need your oral care products to be retail-ready? White-label manufacturing wins at speed.
- Big-box, export, and pharmacy channels prefer OEMs or vetted contract partners because of the stricter documentation and local registrations under this model.
- Products like toothpastes depend on the RDA levels, tube supplier control, and seal integrity. Hence, go for OEM for validated packaging.
- Switching manufacturing models later costs money and time. So be strategic in choosing the initial model, and not as a temporary workaround.
You can send us your specification sheet, and within 48 hours, you will get a model-fit recommendation from us.
What Each Manufacturing Model Really Means (with real-world examples)
OEM (Original Equipment Manufacturer)
Definition: Here, you give the OEM-valmistaja your design and exact specs, and they develop, tool, and produce it for you.
When to choose:
- When you have a unique formula, special claims, or expect high volumes.
- Choose OEM when you must control RDA (toothpaste abrasivity), tube supplier specs, flavor stability for mouthwash, or propellant choice for breath spray.
Example: If a client needs unique active ingredients or packaging, we rule out white-label from the start. We partner with them as an OEM to make low-RDA toothpaste formulations and insist on ISO/ADA testing before approving any toothpaste tube. In the end, the brand owns the formula, claims, and IP, while we, their manufacturing partner, provide R&D, tooling, and scale-up.
Hyvät puolet: Full control, IP protection, and better profits at scale.
Haittoja: Higher startup cost, longer lead times, heavier QA/audit burden, and larger MOQs.
White-Label / Private Label
Definition: The supplier/white-label company sells ready-made products to you, and you label them as yours.
When to choose:
- Often used when you want to launch fast, test the market, or sell simple/commodity SKUs.
- For oral care brands, only buy white-label toothpaste after confirming recent RDA reports and that the tube/label has been pre-validated and will work for your market.
Example: We once worked with a startup as a private label supplier to rebrand an existing whitening paste, and they launched multiple SKUs within weeks.
Hyvät puolet: Fast and cheap to start; low MOQ options.
Haittoja: Hard to stand out in a competitive market, and margins are smaller.
Sopimusvalmistus
Definition: You supply the formula; the manufacturing partner fills, seals, and packages it. You retain IP while your partner runs production.
When to choose:
- You already have a tested formula, but no factory, or you want to avoid plant CAPEX.
Example: A dental clinic with an in-house formula contracts a packer to produce tubes and provide batch COAs.
Hyvät puolet: Keeps your IP and formula control without building a plant
Haittoja: Needs tight QA handover and clear contractual responsibilities. Higher per-unit cost at low volumes.
For more details, download the OEM vs White-Label Comparison PDF.
When to Choose OEM vs White-Label vs Contract Manufacturing
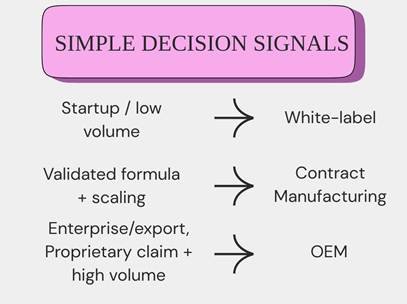
Choose White-label if:
- You need the fastest time-to-market and low upfront cost.
- You’re testing product-market fit or launching a small set of SKUs.
- The product is basic (commodity whitening and freshness) with no regulated claims.
- Must-have checks: The manufacturer provides recent RDA reports (toothpaste), verified tube compatibility, and localized labels.
- Tradeoff: Low differentiation and slimmer margins.
Choose Contract Manufacturing if:
- You already own a validated formula but lack filling capacity.
- You want to keep IP without building a factory.
- You need to scale volumes quickly without heavy CAPEX.
- Must-have checks: The contract clearly states who pays/owns the RDA, includes burst and stability tests, and specifies who handles packaging liability.
- Tradeoff: Needs tight QA handover; per-unit cost is higher at low volumes.
Choose OEM if:
- You want proprietary claims, unique actives, or exclusive packaging.
- You plan for high volumes and long-term margin control.
- Product complexity is high (low-RDA sensitivity paste, mouthwash with unique flavor chemistry, breath-spray propellant choices).
- Must-have checks: Before we sign an OEM contract, we always ask: ‘Who will pay for RDA testing if the first batch fails?’ and we negotiate that up front. We also discuss the full R&D plan, tooling costs, accredited lab testing, and strict IP/QA clauses.
- Tradeoff: Higher setup cost, longer lead time, and larger MOQ.
Rule of thumb: Pick for speed, control, or cost; and document who will pay for tests, handle failures, and own the IP before you sign.
Questions to Ask Before Choosing a Business Model
Questions to Ask BEFORE you Commit to an OEM
- Who owns the formula and IP after development?
- What are the tooling/setup costs, and who pays them?
- What is the MOQ, and where are the volume breakpoints for unit-cost drops?
- What is the full development and validation timeline (R&D, RDA, stability)?
- Will test reports cite method and lab accreditation (ISO/IEC 17025) and include raw data?
- What tube or packaging suppliers are required, and who vets them?
- Which contract clauses cover IP assignment, audit rights, warranties, recall costs and non-conformance remedies?
Questions to Ask BEFORE Purchasing White-Label / Private Label Units
- Is the formula exclusive, or is it sold to other customers?
- Can the supplier provide recent COAs and RDA reports for the SKU?
- Are labels and translations provided, and who owns local regulatory filings?
- What packaging options exist, and has the SKU been tested on our preferred tube/carton?
- What are the MOQs and lead times for reorders and for scaling?
- What change request process exists (artwork, minor formula tweaks) and associated costs?
- Who is liable for product failures or recalls on white-label stock?
Questions to Ask BEFORE Handing Over to a Contract Manufacturing Company
- Do you accept client-supplied formula, and will you sign an IP/ confidentiality agreement?
- What is the filler line capacity, scheduled availability, and changeover time?
- How do you handle sample retention and batch COAs? What is retained and for how long?
- Who funds and owns RDA, burst and stability testing for filled product (and re-tests)?
- Who supplies/qualifies tubes and closures, and who owns packaging liability?
- What are the changeover and rework costs if we change the tube type or artwork?
- Do you allow customer audits, third-party lab sampling, and access to QC records?
Practical Case Scenarios — Which Manufacturer to Choose
Scenario A — Lean startup: Travel kit test (3 SKUs)
Founder: Amina is the owner of a small wellness brand. She launched a 3-SKU travel kit (toothpaste, mini mouthwash, breath-spray) to test pop-up retail at airport kiosks, and she had only a few thousand dollars to spend. We chose white-label toothpaste and mini mouthwash because they cut development time to 6 weeks and cost under $5K. Her order included recent RDA reports for the toothpaste, a tube-compatibility test, and propellant compliance papers for the breath-spray.
Result: Sales were quick, and she had low upfront spend. The trade-off was that the same formulas were sold to competitors.
Lesson: White-label works for fast tests; however, plan an exit path to contract fill or OEM manufacturing if volume grows. In her case, we planned to shift to a contract fill once we validated demand.
Scenario B — Growth stage: Validated formula, scaling to 75k/yr
Yritys: BrightSmile is a B2C oral-care brand. Year 1 sales proved demand, and they decided to add an enamel-repair claim and expand their sales channels. They kept the formula and moved to a contract manufacturing model to meet their volume targets. The contract spelled out who pays for RDA, burst and stability tests, and required batch COAs and sample retention.
Result: No CAPEX, better margins as volume rose, and they kept IP.
Lesson: Contract manufacturing is ideal when you own the formula but need production capacity. However, get test ownership and liability in writing.
Scenario C — Enterprise: National retail + export
Retailer: HealthMart wanted an exclusive “sensitivity” line for their local shelves and exports. They commissioned us to develop a unique low-RDA paste and custom laminated tube. Our team found that they needed tooling and a larger MOQ, yet we delivered ISO/ADA RDA reports to substantiate their claims, and also validated their packaging for transit.
Result: They secured exclusivity, got a higher shelf margin, and smoother retailer approvals. Lesson: Choose OEM for strict IP controls, exclusive claims, and large-scale retail. Keep in mind you also need a budget for development time and a higher MOQ.
Still not sure which manufacturer type fits your brand and business stage? Send us your spec sheet for a model-fit recommendation (48-hour response).
Due Diligence Checklist (What to Verify with Any Supplier)
Operational checks (Quick Things to Confirm)
- Can they actually produce your volume?
- Do they run quality systems?
- Do they keep samples and records?
- Who pays for the testing?
- Which tube or closure supplier do they use?
- What are their MOQs, unit-cost breakpoints, and tooling/changeover costs?
Legal & IP (Must-have Contract Items)
- Who owns the IP/formula?
- Are there NDAs in place?
- Who pays for tooling?
- Who pays for recalls, rework, or credits if batches fail?
- Will they allow you to audit or use a third-party lab? *You must be able to do this.
Regional & Market Checks
- Who files registrations and provides translated artwork for target markets (FDA/EU, etc.)?
- Hope they provide COO, importer details, and artwork that meets local rules?
- Does the market require extra local tests that add time?
Red Flags in Choosing a Manufacturer Type for Your Oral Care Brand
Stop if you see any of these:
- No lab accreditation.
- Vague or missing acceptance criteria.
- Refusal to sign IP/tooling clauses.
- No sample retention or COAs.
- Cannot show recent RDA or burst test data.
Yleiset virheet vältettävät
- Buying purely on price without checking QC.
- Not defining who pays for re-tests.
- Skipping tube/closure compatibility runs.
- No sampling plan or acceptance criteria in the RFQ.
- Failing to budget for retesting and retooling if you change the model later.
Johtopäätös
Choosing a manufacturing model goes beyond price, MOQs, and IP ownership; it is a business choice and should be treated as a strategic one. OEM works best if you want to retain proprietary claims and for high-volume orders. Pick white-label if you need to launch fast with low cost. Contract manufacturing is best if you already have a tested formula but no factory.
Before you sign with any brand, agree on measurable acceptance limits (RDA, burst, stability), decide who pays for and owns each test, and put remedies (rework, credit, recall steps) in the contract. Your product becomes “Retail-ready” when the product is tested, paperwork is complete, packaging is validated, and labels are approved; otherwise, you risk product delays or rejection.
Next steps: If you need help choosing a model, book a call with our procurement team, download the Model Comparison PDF (with XLSX calculator), or request a Sample Kit (includes RDA + burst reports) to get started on your model selection.
Frequently Asked Questions about OEM, White Labelling, and Contract Manufacturing
- What is the difference between contract manufacturing and private label?
In contract manufacturing, you supply the formula, while the manufacturing partner produces the product. In private-label manufacturing, the manufacturer/supplier provides finished SKUs, then you rebrand them.
- What is the difference between white label and OEM?
White-label manufacturing means already manufactured products are rebranded. OEM means the products are customized to your proprietary specifications with R&D and tooling by a manufacturer.
- What is the difference between OEM and contract manufacturing?
The OEM company is responsible for product development and full manufacturing using the specs you provide. In contract manufacturing, you give the manufacturer “your formula”, and they fill and finish the production.
- Is white-label better than private-label?
White-label and private-label refer to the same manufacturing models; hence, neither is better than the other.
- Does the product type determine which model you will use for manufacturing?
Yes, it does. Products like toothpastes, mouthwashes, and breath sprays that require regulated claims, RDA, flavor stability, or propellant rules often require OEM or contract manufacturing rather than white-label.
- What are the contract essentials that procurement teams should keep in mind?
Items that should be in the contract include IP/tooling ownership, MOQ, lead times, QC acceptance (RDA, burst), test ownership, liability, remedies, and audit rights.
- Can a brand switch models later?
Yes, but you may need to retest and retool, new MOQs, label updates, and possibly fill supply gaps.
- What clauses must appear in OEM contracts?
Clauses to include are IP assignment, tooling costs, exclusivity, acceptance criteria, warranties, recall indemnity, testing responsibilities, and audit access.







