After production comes sales and marketing. What makes toothpaste retail-ready is an aggregation of tests and compliance checks, such as the toothpaste RDA, burst tests, and labeling. Without getting these checks right, you risk falling into major issues like seal failure, high RDA, mislabeled country of origin, and non-compliance with regional rules, among others.
In this article, we detail factors in the toothpaste manufacturing process that could lead to product recalls, the risks that follow, and how to avoid them.
Punti chiave
- A retail-ready toothpaste is measurable. In one project, we went the extra mile by securing RDA certificates from two different accredited labs to reassure a risk-averse retailer. That decision cost more in lab fees, but it expedited listing approval because the buyer trusted the results.
- Bad packaging is a leading cause of rejected units and product returns. Do checks on the tube material, seal strength, and tube leak/burst.
- To avoid delays at customs, properly label your toothpaste. Include a correct ingredient list and names, translations, and importer/manufacturer details.
- Your compliance packet should include a sample kit, COA, RDA & burst reports, MSDS, and a stability summary.
The Toothpaste Production Process
Before contracting a supplier, your procurement and marketing teams must confirm that these core steps and documents are in check:
- Formulation & ingredients: List all the toothpaste ingredients and specify abrasive type and target Relative Dentin Abrasivity (RDA). State active ingredients and acceptable pH.
- Ingredient handling: Get the COAs, lot traceability, and storage conditions for temperature- or humidity-sensitive materials.
- Mixing & consistency: Define process stages (dry/wet), mixer type, target texture/flow, and controls for temperature and air removal to retain product consistency.
- Quality testing: Obtain RDA/abrasivity reports, microbial limits, pH and viscosity checks, preservative efficacy, tube-burst tests, and accelerated + real-time stability data.
- Tube flling & packaging: Select the tube material (laminate, plastic, aluminium) based on barrier and burst strength. Note the filler calibration, seal integrity, inline leak/burst checks, batch coding, and rework/rejection rules.
- Toothpaste labeling: Final label should show ingredient names (INCI), net quantity, manufacturer/importer, country of origin, and warnings; include translated labels where needed.
- Distribution & Docs: Confirm MOQ, lead time, palletization, and customs requirements. Include a compliance packet: COA, MSDS, RDA, and burst reports, and stability summary to support retailer listing and clearance.
Getting the toothpaste ready for retail means these steps and documents are in place. Below are some factors that often lead to delays in the product listing and toothpaste retail and how to handle them.
RDA Scoring Explained
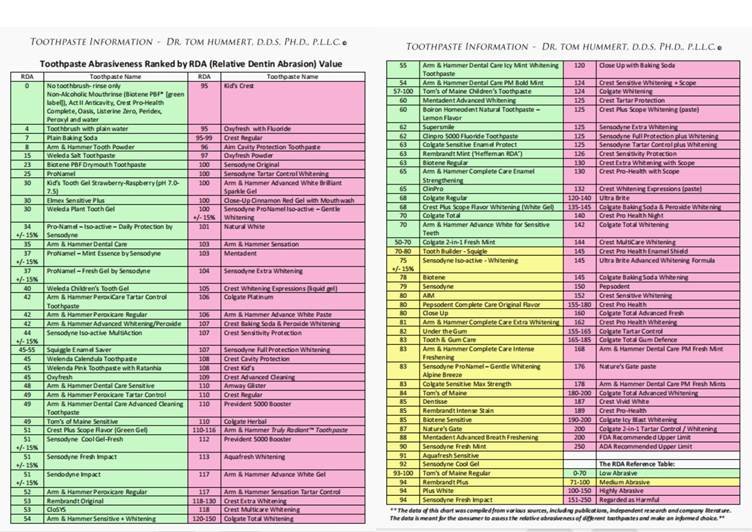
Relative Dentin Abrasivity (RDA) measures how abrasive a toothpaste is to the dentine (the softer, inner tooth layer) versus the enamel (the harder and more resistant part).
Toothpastes are ranked on their RDA levels: low (0 – 70), medium (71 – 100), high (101 – 150), and harmful limits (151 – 250). Toothpastes with very high RDA levels ‘scour’ away the dentin and expose the enamel, predisposing it to major oral health risks. These risks ultimately affect your product claims, warranty, and acceptance by retailers. To the end users, this will look like increased complaints and return rates, and a risk of the toothpaste getting delisted.
The RDA level you should go for in producing your toothpaste primarily depends on the target of the product. Most whitening toothpastes fall into the medium to high and high RDA levels, while toothpaste for sensitive teeth often runs lower. However, you should consider the acceptable RDA levels in the market you wish to explore in your toothpaste production process.
For instance, the maximum RDA a toothpaste should have (to sell in the US) according to the FDA is 200. Most toothpastes in the US market are within the 100 range, however charcoal toothpastes are higher than 100. For the EU market, the acceptable RDA upper limit (as defined by the ISO) is 250.
Recently, when formulating a whitening paste, we aimed for RDA ~110. But after lab tests came back at 130 (still under the US 200 limit), our marketing lead argued for pulling back to RDA 90 to claim “sensitive friendly.” We ultimately split the line into two products: one high-RDA whitening paste (with a caution label) and one true low-abrasive sensitive paste. This split was a deliberate exclusion/segmentation strategy driven by test results and market positioning.
RDA testing is done in standardized labs. Here, they compare the toothpaste in review to a reference abrasive using prepared dentine samples. Controlled brushing or polishing is done, and the worn material from the dentin is collected. The RDA value is then calculated against the reference. Your toothpaste brand is ready for the market if it stays within the required RDA limits. Hence, you should state it in the contracts, along with the accredited lab reports, test methods, and sampling plans.
Packaging Burst Test Demonstration — Toothpaste Tube Test

The packaging burst test of a toothpaste checks the tube’s ability to resist internal pressure and seal failure during filling, transport, and temperature changes.
The toothpaste tube passes the test if it doesn’t split open, has no visible leakage at the seal, and the seal remains tight under the agreed tolerance level for that sample. Whereas a failed test could look like a ruptured tube, seepage of the content, or seal delamination.
We once rejected a batch of tubes on the line because a lamination defect caused minor leaks. Correcting the supplier (and paying for upgraded material) increased our unit cost by a few cents, but it saved thousands in potential returns and brand damage.
Burst pressure testing can be done in different ways, including:
- Burst threshold test: The pressure inside the tube is continuously and uniformly increased until it fails (bursts).
- Hold or creep test: Sustained pressure is applied to the tube to see if it fails after a set duration.
- Creep test to failure: A constant internal pressure is held until the tube eventually bursts.
- Combination tests: The tube is held at a set pressure for a short time, then increased until the tube bursts.
The pressure medium can be:
- Pneumatic pressure: The tube is continuously inflated with air until it fails (bursts).
- Hydrostatic pressure: A liquid (eg, water) is pressurized in the tube until it bursts the tube.
A retail-ready toothpaste tube should have peel and seal strengths that meet supplier-agreed thresholds. The seal/peel strength tests measure the strength of the toothpaste lid/seal. It should also not leak during the storage or hold period.
This test is so important, that an EU retailer rejected 18,000 toothpaste units because the tube seal failed under drop test. Packaging failure is also one of the leading causes of Amazon negative feedback, and you definitely don’t want your brand name on those reviews.
Send us a request to get a sample burst-test report for your toothpaste tubes.
How Toothpaste Labeling Affects Customs Clearance & Retail Listing
In the U.S., your toothpaste label must show the:
- Active & inactive ingredients
- Indications
- Warning statements
- Manufacturer information
- Directions for use
Generally, food and drug items (including toothpastes) produced for export are required to have labels that meet the following criteria:
- All information should be accurate and not misleading.
- The label must be in the official language(s) of the destination market.
- The brand name must be clearly stated.
- Ingredient list (with allergens declared), net content of the product, the name and address of the manufacturer or distributor, and the country of origin must be clearly indicated.
- The batch or lot number, date of manufacture, and an expiry or “best before” date, storage and use instructions, and regulatory information, such as a registration number.
It is the toothpaste labeling that determines whether your products will clear import inspections and get to your retailers. Misbranded or noncompliant toothpastes can be impounded, and detained goods may require reconditioning after a specific timeframe or be refused entry.
If space is tight, we sometimes use a QR code (where regulations allow) to host detailed ingredient lists, which keeps the front label clean. P.S. We used this solution in a market with dense language requirements.
The common toothpaste labeling pitfalls that trigger detention at customs are:
- Unclear ingredient names
- Missing importer or country-of-origin details
- Unsupported product claims
- Incorrect net quantity
- Untranslated mandatory information for target markets.
R&D & Compliance Documentation That Brands Need Before Listing
A retail-ready toothpaste must have clear, verifiable documents on the entire product lifecycle, from R&D to manufacturing and labeling. These ensure that the product is safe and marketed within legal limits. A fully compliant toothpaste is far more likely to gain approval from major retailers, online marketplaces (eg, Amazon), and regulators.
The minimum dossier for a retail-ready toothpaste contains (required per SKU):
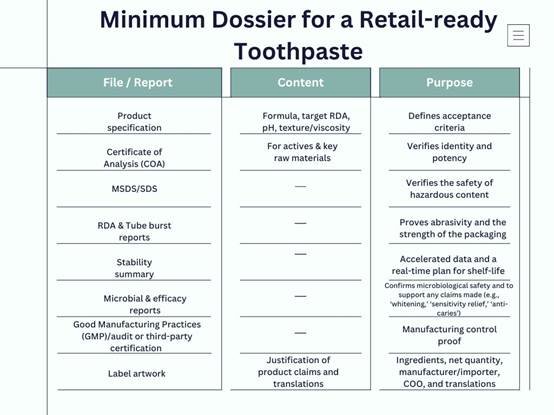
Over the years, we’ve learned that keeping everything organized is half the battle. For each SKU, we create a “dossier folder” with scans of every COA, stability report, and test certificate. In one case, a retailer audit wanted evidence of microbial limits on an older SKU. Because we kept the raw lab data, we were able to quickly extract the necessary charts. Without that quick access, we would have missed the listing window.
The key regulatory standards to reference include:
- USA: FDA standards on cosmetics/over-the-counter drug use.
- EU: CE/market access requirements and safety standards.
- International: ISO standards (e.g., ISO 11609, ISO 9001) and third-party test labs (SGS).
How to package documents for RFQs and retail submissions
- Create one Compliance Packet PDF and name files clearly, e.g., SKU_TestType_Date.pdf, and include a table of contents. We name sample-kit PDFs this way (e.g., MINT-WH-001_COA_2025.pdf) so that dozens of PDFs don’t get mixed up. In practice, we’ve seen suppliers send generic file names like report1.pdf, and it creates confusion. This small upfront step saves time on the retailer side.
- We also include a one-page “executive summary”, listing each test result’s pass/fail status, dates, lab accreditation, and contact for technical queries. In one instance, an Amazon vendor checklist only skimmed the summary page, so having concise results there sped up approval.
Timeline & sample-kit (expectations)
- Request a starter sample kit: 3–5 finished tubes, COAs, RDA, burst test photos, and a stability summary.
- Expect accredited lab turnaround of 2–4 weeks for RDA and burst tests. Stability reports take longer (accelerated data in weeks, full real-time in months).
- Build these lead times into RFQ deadlines and acceptance windows.
We always pad the schedule. For instance, if the lab says ‘RDA in 2–4 weeks’, we plan for 4 weeks. Last-minute shipping delays of samples are common. Sometimes we go for the fastest lab (even at a higher cost) to prevent delays, but of course, depending on a brand’s priorities.
Not sure where to start? Download our Retail-Ready Compliance Checklist (PDF) to kickstart your toothpaste production process.
Why Your Toothpaste Formulation Matters and Why Getting Your Product Ready for Retail is Important
Your formulation determines product performance, category, and cost structure. It determines whether the product cleans, whitens, or soothes sensitivity; which raw materials you must source; how complex production will be; and how much testing is required. A poorly specified formula leads to inconsistent batches, retailer rejections, higher return rates, and hidden costs for rework or recalls.
Business Implications
- Performance = trust: Repeatable results reduce customer complaints and protect shelf placement.
- Cost & margin: Specialty actives and higher-spec packaging increase unit cost and affect pricing.
- Time to market: New claims need more validation and longer stability programs.
- Channel fit: Some retailers and export markets require extra data or certifications.
Quality control checklist
- Define measurable acceptance criteria in the spec: RDA target, pH range, viscosity, microbial limits, preservative efficacy, and shelf-life endpoints.
- Ask your produttore di dentifricio for batch COAs, lab accreditation results, and sample retention for destructive tests (e.g., tube burst).
- Set sampling plans, acceptance thresholds, and contract remedies (in case of rework, credit, rejection).
- Ensure label claims match test data and ingredient concentrations.
Practical next steps for procurement
- Include QC acceptance criteria in RFQs.
- Ask for supplier audit evidence and third-party lab reports.
- Factor testing and sample runs into cost models.
Clear toothpaste formulation control reduces listing risk, protects margins and preserves brand reputation.
Conclusione
A retail-ready toothpaste should have measurable product specs, valid packaging documents, and a complete compliance dossier that includes the target RDA, COA, MSDS, stability data, label proofs, burst data, and seal data.
Before you launch your toothpaste brand into the market, confirm who is responsible for label compliance and customs clearance, ask for raw test data and lab accreditation, and make contractual agreements in case any non-conformance arises.
Partnering with a toothpaste manufacturer who supplies sample kits, RDA and burst test certificates, COAs, and finished-product stability summaries shortens your toothpaste approval timelines and reduces listing risk. Retail-ready means tested, documented, packaging-validated, and pre-approved for labeling, otherwise, the risk of rejection and product failure remains high. If you’re ready to move from concept to shelf, download our Model Comparison PDF and Toothpaste RDA Matrix or contact our RFQ team to request a sample kit and compliance packet tailored to your SKU.
Domande frequenti
- What is RDA in toothpaste?
RDA (Relative Dentin Abrasivity) is a lab metric that measures how abrasive a toothpaste is to the dentine (the softer part of the tooth). It is reported as a single number, with a higher RDA meaning the toothpaste is more abrasive.
- How do you test RDA for toothpaste?
RDA testing is done with standardized lab methods (ISO 11609). A human or animal (cow) tooth is extracted, irradiated, stripped of its enamel, and placed in a brushing machine. This machine brushes the tooth according to ADA standards, and the radioactivity of the rinsed water is measured.
- Why are abrasive ingredients added to toothpaste?
Abrasives such as silica and calcium carbonate are added to toothpaste to remove plaque and surface stains mechanically.
- What packaging features increase toothpaste appeal on retail shelves?
Clear brand name and product details, strong color contrast, readable claims, tamper-proof seals, and shelf-friendly carton or hang-tab formats make toothpaste more appealing on retail shelves.
- How do toothpaste brands ensure their products meet retail display standards?
Toothpaste brands ensure their products meet retail display standards by validating the unit and carton dimensions and materials, barcodes/GTINs, and shelf trays with the retailer specifications. Provide shelf mockups and check for pallet stability and carton stability.
- How to choose packaging partners for retail-ready toothpaste production?
Go for partners with tube capability, barrier expertise, QC systems, testing labs or lab partners, and documented lead times and MOQs. Verify their certificates, sample turnaround, and willingness to provide burst/seal test data and packaging validation.
- Can brands reuse old test reports?
Yes, they can. Brands can reuse old test reports if the report covers the same formula, same production site and line, identical packaging, and is recent enough per retailer/regulator requirements. If the formulation, supplier, process, tube material, or a significant time (>12–24 months) has passed, a retest is required.
- Who pays for testing?
Typically, the brand (buyer) pays for the initial R&D and pre-production testing and manufacturers often include routine batch QC in unit costs. However, you can negotiate test responsibilities and cost-sharing in the contract and clarify who pays for failure re-tests or third-party verification.
- HDPE tube vs laminate tube – which is more “retail-safe”?
Laminate and multi-layer tubes are generally stronger, hence they have a better burst/seal performance and are more “retail-safe”. HDPE tubes are robust and recyclable, but offer a lower barrier and different sealing behaviour. Your ideal packaging material also depends on the compatibility testing and retail requirements.







