Introduction
In oral care, especially whitening products, viral traction often accelerates demand faster than manufacturing, packaging, and regulatory systems can absorb. This gap is where most high-growth products fail—not due to formulation flaws, but because operational risk compounds faster than commercial momentum.
For importers, DTC founders, Amazon sellers, and retail sourcing teams, this case matters because it shows what actually breaks—or scales—when viral demand collides with retail reality.

This OEM oral care case study documents how a TikTok-viral whitening mouthwash transitioned from unpredictable DTC demand to national big-box retail placement through OEM-led execution. The product scaled production volume by 12× within nine months, while reducing transit leakage from approximately 6% to below 0.2%, without sacrificing margin, compliance integrity, or retail timelines.
Note: The brand featured in this case study has been anonymized to protect commercial confidentiality and avoid legal or contractual disclosure issues.
Initial Conditions — What ORALABX Assessed Before Accepting the Project
- OEM assessment perspective:
- The brand owned marketing, positioning, and demand generation. Oralabx owned manufacturing execution, packaging engineering, compliance systems, and retail-readiness delivery.
- SKU profile:
- Whitening mouthwash (liquid oral-care SKU with elevated compliance exposure.)
- 237 ml resin bottle.
- Sales channel:
- TikTok-driven DTC launch.
- Early-stage example of TikTok viral product scaling without retail infrastructure.
- Demand pattern:
- Unpredictable, spike-driven volume.
- No stable reorder cadence or forecasting baseline.
- Initial risks identified by OEM and procurement impact:
- Bottle leakage under transit pressure
- Procurement impact: leakage increases chargebacks, returns, and delays retail readiness oral care approval.
- Whitening claim sensitivity
- Procurement impact: claim misclassification risks, forced relabeling, and delays regulatory approval for mouthwash OEM manufacturing.
- No batch history or traceability system
- Procurement impact: Lack of records increases audit exposure in a private-label mouthwash case study environment.
- Packaging not retail-compliant
- Procurement impact: Failed ISTA transit testing results in lost retail slots and stalled TikTok viral product scaling.
- OEM principle reinforced:
This OEM oral care case study demonstrates that viral demand amplifies operational, compliance, and documentation risk faster than formulation risk—making OEM execution decisive during scale.
What Went Wrong First

Early Production Missteps
- Initial MOQ request misaligned with sell-through reality
- OEM issue: production volumes were committed before demand signals stabilized, a frequent failure in the early-stage MOQ staging strategy for viral products.
- Commercial risk: excess inventory tied up as working capital, reducing flexibility during retail readiness oral care evaluation.
- DTC packaging unsuitable for retail transit standards
- OEM issue: packaging design met direct-to-consumer needs but not the durability requirements expected in mouthwash OEM manufacturing for brick-and-mortar distribution.
- Commercial risk: failed drop and transit testing delayed retailer approval, weakening momentum in this private-label mouthwash case study.
- Lead time mismatch between marketing velocity and manufacturing capacity
- OEM issue: marketing-driven demand accelerated faster than production capacity could scale safely, a common constraint in TikTok viral product scaling.
- Commercial risk: a three-week delay nearly resulted in a missed retail launch window, directly threatening retail readiness oral care milestones.
Commercial Impact
- Three-week production bottleneck
- A temporary capacity constraint slowed output just as demand accelerated, compressing retail readiness timelines and reducing margin for error ahead of retailer onboarding.
- Risk of over-producing printed packaging
- Packaging artwork and print volumes were approved before the MOQ staging strategy and compliance requirements were fully stabilized, creating unnecessary inventory exposure.
- Near-miss on retailer documentation deadline
- Batch records and compliance files were finalized under time pressure rather than through a standardized OEM workflow—nearly derailing retail approval.
OEM Takeaway Callout:
Scaling too fast creates larger downstream losses than scaling deliberately—especially in oral care, where rushed TikTok viral product scaling magnifies packaging risk, documentation gaps, and retail readiness oral care failures faster than it increases revenue.
Mid-Article CTA #1: Request MOQ Staging Plan
Planning your next scale step, but unsure how much volume to commit? Request an OEM-built MOQ staging plan that maps pilot batches, intermediate runs, and retail-ready production—so you can protect cash flow, avoid packaging write-offs, and scale only when demand and compliance risk are validated. (www.ruiqigo.com)
OEM-Led Staged MOQ Model
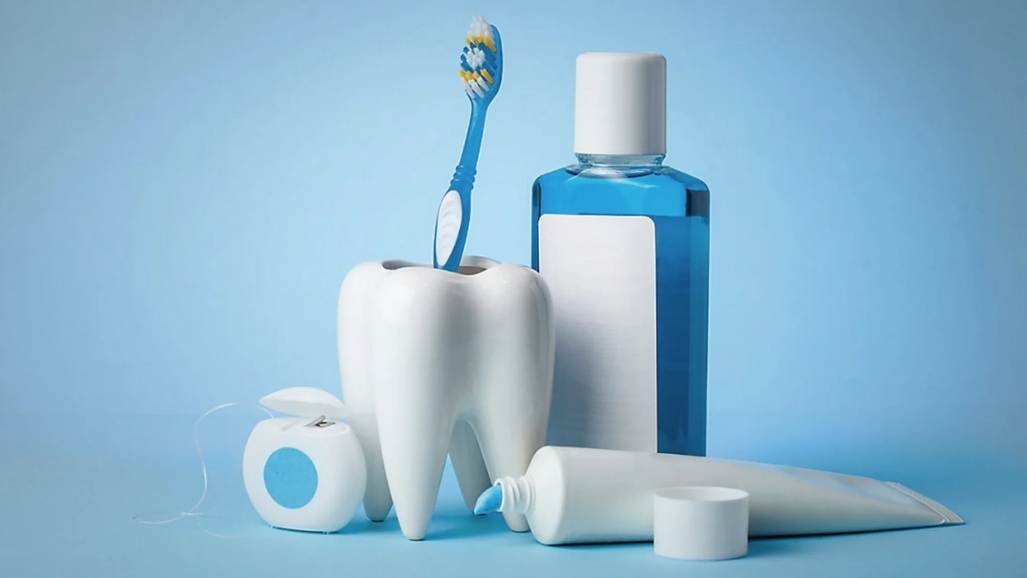
Pilot Batch (Validation Phase)
- Pilot MOQ:
- 5,000 units, intentionally limited under a controlled MOQ staging strategy to support disciplined scale-up.
- Purpose:
- Validate short- and mid-term stability required for retail readiness oral care, and downstream buyer audits.
- Monitor color shift under varied lighting and storage conditions, a known risk during TikTok viral product scaling, where visual performance drives consumer trust.
- Confirm aroma consistency and batch repeatability within a compliant mouthwash OEM manufacturing environment.
- Conduct leak testing to evaluate transit durability ahead of retail and export distribution.
- Result:
- Formulation approved for scale within this OEM oral care case study.
- Packaging flagged for redesign before advancing volume commitments.
- Procurement impact:
- Pilot-scale execution prevented six-figure packaging exposure and reduced audit risk, reinforcing why staged validation is essential in any private label mouthwash case study transitioning from DTC to retail.
Intermediate Scale Batches
- Scaled MOQ:
- MOQ increased to 25,000 units once pilot data supported a sustainable MOQ staging strategy for viral oral-care products.
- Operational improvements:
- Lead time reduced from 42 days to 28 days by synchronizing production planning with more predictable reorder behavior, an inflection point typical when moving from DTC virality to early retail readiness.
- Batch sheets and CoAs are standardized to support oral care regulatory approval workflows and downstream retailer audits.
- Processes formalized within a repeatable mouthwash OEM manufacturing model for retail scale.
- Inventory control:
- Production volumes aligned to rolling forecasts rather than peak-demand assumptions.
- Inventory risk was actively controlled while maintaining momentum during TikTok viral product scaling.
- Procurement impact:
- Enabled predictable replenishment cycles without locking excess cash early—a key requirement for procurement teams managing retail transition risk in private label mouthwash programs.
Retail-Ready Production
- Final staged MOQ:
- MOQ increased to 100,000 units only after stability, packaging performance, and documentation systems were validated through earlier phases, completing the MOQ staging strategy for retail-scale oral care.
- Capacity commitment:
- A dedicated production slot was introduced once demand and compliance risk were sufficiently predictable, aligning with best practices in mouthwash OEM manufacturing for big-box retail.
- Workflow optimization:
- Packaging execution and regulatory documentation were run in parallel rather than sequentially, supporting faster approvals within oral care regulatory submission timelines.
- This structure reduced bottlenecks common when scaling from DTC into retail-ready private label mouthwash programs.
- Procurement impact:
- Protected retailer onboarding schedules and minimized launch risk during high-volume scale-up, reinforcing the importance of OEM-led systems in managing retail readiness for viral oral-care SKUs.
Mid-Article CTA #2: Request Retail-Ready Documentation Pack
Download a sample (anonymized) documentation pack showing exactly what retailers approved in this case. (www.ruiqigo.com)
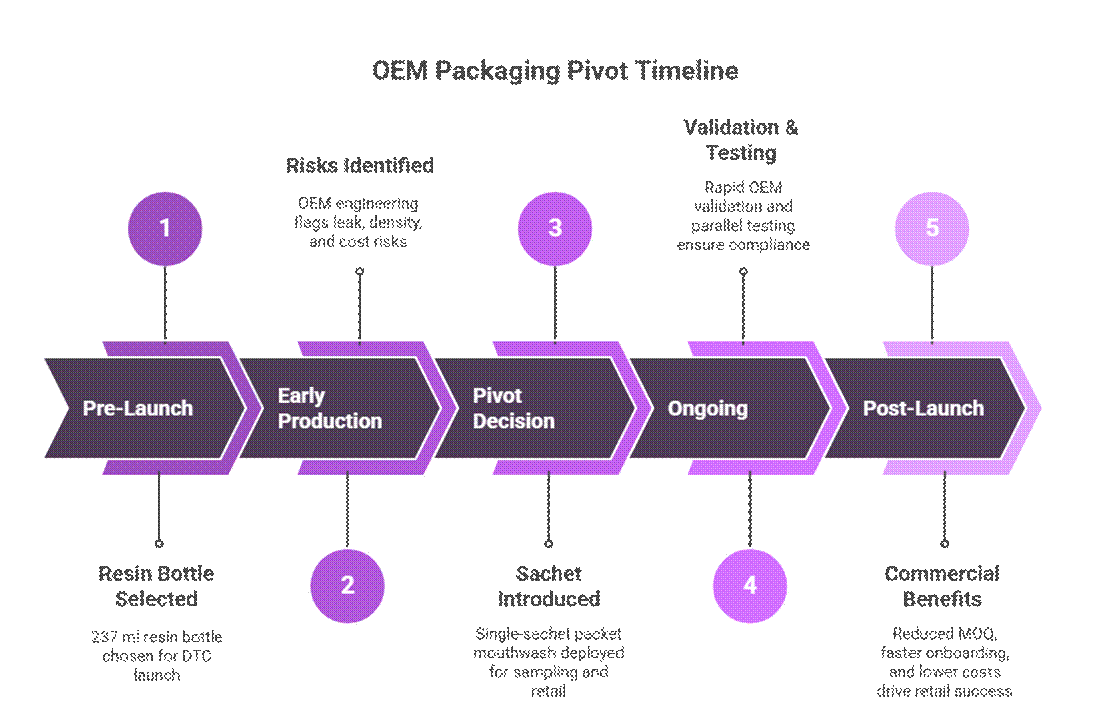
Packaging Pivot — OEM Engineering Decision

Original Format (Risk Profile)
- Packaging format:
- 237 ml resin bottle originally selected for DTC launch.
- Identified risks from an OEM engineering perspective:
- High leak risk under compression and vibration during palletized transit, a known failure point in mouthwash OEM manufacturing for retail distribution.
- Poor pallet density, limiting carton efficiency and increasing damage exposure during retail readiness oral care logistics.
- Higher freight cost per unit, reducing margin flexibility when transitioning from DTC to retail-ready private label mouthwash programs.
- OEM implication:
- While acceptable for small-batch DTC shipping, this format introduced compounding cost and risk at scale, reinforcing why packaging engineering is central to controlled TikTok viral product scaling.
OEM Packaging Pivot
- Packaging format introduced:
- Single-sachet packet mouthwash deployed as a launch and sampling format alongside the core SKU.
- Engineered to support controlled scale within a retail-ready private label mouthwash program.
- Strategic use cases enabled:
- Sampling: reduced risk exposure while validating consumer response during TikTok viral product scaling.
- Retail buyer approval: provided a low-risk, compliant format to support retail readiness oral care reviews.
- TSA-friendly DTC kits: enabled lightweight, travel-safe bundles without introducing leakage risk.
- What only a strong OEM could solve:
- Rapid validation of a new packaging format within mouthwash OEM manufacturing systems.
- Parallel testing and documentation were executed without delaying core SKU production, preserving momentum while reducing downstream risk.
Commercial Impact
- Reduced MOQ exposure:
- Single-sachet packaging allowed early retail engagement and sampling without committing to high bottle MOQs, reinforcing a disciplined MOQ staging strategy for retail-ready oral care.
- Faster retail onboarding:
- Low-risk, leak-resistant formats simplified transit testing and buyer review, accelerating approvals within mouthwash OEM manufacturing workflows.
- Lower landed cost for trial units:
- Improved pallet density and reduced freight weight lowered per-unit landed costs, supporting efficient trial programs during TikTok viral product scaling and private label mouthwash retail launches.
Regulatory & Testing Execution (OEM Methodology)
Whitening Mouthwash-Specific Testing
- Color stability testing:
- Evaluated under varied lighting and storage conditions to account for viral lighting sensitivity during TikTok viral product scaling.
- Ensured visual consistency required for retail readiness oral care approval.
- Aroma drift testing:
- Monitored fragrance stability across production runs to maintain sensory consistency within mouthwash OEM manufacturing systems.
- Microbial load reports:
- Conducted routine microbial testing to meet oral care regulatory approval expectations for liquid products with daily use exposure.
- Claim-risk review (cosmetic vs. OTC):
- Assessed whitening and functional claims to prevent misclassification and forced relabeling in private-label mouthwash retail programs.
- Procurement impact:
- Early, SKU-specific testing prevented post-launch compliance rework, protecting timelines and margins during retail scale-up.
Documentation Pipeline
- Structured OEM documentation flow:
- Bench testing → Stability studies → Batch sheets → Certificates of Analysis (CoA) → Export documentation.
- Built as a repeatable pipeline within mouthwash OEM manufacturing to support retail readiness oral care reviews.
- Retail-approved submission pack:
- All technical, quality, and compliance documents assembled into a single, retailer-ready submission pack.
- Designed to reduce back-and-forth during buyer review and support oral care regulatory approval in private-label mouthwash programs.
- Procurement impact:
- A standardized documentation pipeline minimized approval delays and reduced audit exposure during TikTok viral product scaling and retail onboarding.
Why Oralabx (OEM Perspective)
Oralabx specializes in turning volatile demand into retail-ready scale. Unlike generic contract manufacturers, our teams integrate packaging engineering, compliance systems, and MOQ strategy into one execution model—so brands avoid leakage losses, documentation delays, and margin erosion during growth. This case reflects how we operate as an OEM execution partner, not just a production vendor.
OEM Takeaway Added:
Viral products require more testing discipline, not shortcuts—especially in oral care, where accelerated TikTok viral product scaling magnifies regulatory exposure, claim risk, and retail readiness failures if OEM testing systems are not enforced early.
Timeline Compression & Outcomes
- Hero metric focus:
- This phase highlights how OEM-led systems compressed timelines while reducing operational and compliance risk during TikTok viral product scaling.
- OEM execution outcomes (anonymized):
| Phase | Before OEM Intervention | After OEM Execution |
| Lead time | 42 days | 24-28 days |
| MOQ risk | High | Staged |
| Retail readiness | Unclear | Approved |
| Packaging loss | High | Minimal |
| Scale outcome | – | 12× volume in 9 months |
● Procurement impact:
- Timeline compression combined with staged MOQs and packaging discipline enabled predictable retail readiness oral care execution without sacrificing cash control or compliance rigor.
What Procurement Teams Should Learn From This Case
- Viral ≠ scalable:
- TikTok viral product scaling creates demand signals, not operational readiness; without OEM systems, volume amplifies risk faster than revenue.
- MOQ staging preserves cash:
- A disciplined MOQ staging strategy limits inventory exposure while demand stabilizes, especially critical in retail readiness oral care programs.
- Packaging flexibility protects speed:
- OEM-led packaging options reduce transit risk, shorten approval cycles, and prevent delays common in mouthwash OEM manufacturing at scale.
- Dedicated production slots should be timed—not rushed:
- Capacity commitments should follow validation milestones, not marketing pressure, to avoid bottlenecks in private-label mouthwash growth.
- OEM capability determines commercial outcome:
- This OEM oral care case study demonstrates that manufacturing, compliance, and documentation strength—not virality—ultimately decide whether high-growth oral-care SKUs succeed in retail.
Conclusion (OEM Authority Close)
The success of this product was not driven by virality; it was driven by execution. While TikTok exposure created demand, only disciplined OEM systems transformed that demand into durable, retail-ready scale. This OEM oral care case study shows why most brands fail at the DTC-to-retail transition: they scale volume without first engineering a manufacturing strategy, packaging resilience, and regulatory discipline.
FAQs
Q1. Can we scale without locking large MOQ cash early?
Yes. A staged MOQ approach allows brands to validate stability, packaging performance, and documentation before committing capital. In TikTok viral product scaling scenarios, a disciplined MOQ staging strategy protects cash while demand patterns normalize and retail readiness oral care requirements are met.
Q2. Do viral oral-care products require different testing (color shift, aroma)?
They do. Viral exposure increases scrutiny on visual and sensory performance. Color stability under varied lighting, aroma drift across batches, and microbial load testing become more critical in mouthwash OEM manufacturing when products are repeatedly filmed, reviewed, and stored across uncontrolled environments.
Q3. At what point should a brand pay for a dedicated production slot?
Only after formulation stability, packaging validation, and compliance documentation are proven. In private label mouthwash programs, premature capacity commitments often create bottlenecks rather than speed if demand and retail readiness are not yet predictable.
Q4. How do viral SKU surges affect OEM timelines?
Viral demand compresses decision-making but does not compress manufacturing physics. Without OEM-led buffering and sequencing, TikTok viral product scaling can create lead-time conflicts that delay retail onboarding rather than accelerate it.
Q5. Who owns compliance files in a viral product model?
A capable OEM should maintain and control master compliance files, including batch records, CoAs, and stability data. Centralized documentation is essential for retail readiness oral care reviews, and protects brands during audits and retailer submissions.
Q6. When should MOQ staging be locked?
MOQ staging should be finalized only after pilot validation and initial retail feedback. Locking volumes too early increases inventory and packaging risk, especially during high-velocity private label mouthwash scaling.
Q7. Is single sachet packet mouthwash acceptable for retailer evaluation?
Yes. Single-sachet formats are commonly used for buyer review, sampling, and early approval. In this OEM oral care case study, sachet packaging reduced risk, improved logistics, and accelerated evaluation without delaying core SKU production.
Final CTA: Book Scalability Assessment Call
Evaluating OEM partners for a high-growth oral-care SKU?
Schedule a scale-risk audit, request an MOQ staging plan, or download sample documentation to evaluate whether your current OEM setup can withstand viral demand, retail scrutiny, and compliance pressure. Book a scalability assessment: (www.ruiqigo.com ).







